Our projects
Description
For nearly 40 years, we have developed medical and healthcare products ranging from surgical masks to oncology imaging systems. This journey fuels our passion for providing solutions that enhance patients’ quality of life.
View projects
Medical product development
Our multidisciplinary team works in a variety of clinical and healthcare settings
For nearly 40 years, Alto has been developing award-winning medical and healthcare products of varying complexity, from disposable masks to breast cancer imaging systems.
Alto’s expertise in the medical field is focused on product development activities. Our design process, supported by mechanical engineering, feeds your internal verification and validation (V&V) and certification processes. Our activities adapt to your Quality Management System (QMS) to meet Health Canada and FDA regulatory requirements.
Whatever the field of application of medical products, we place the human being at the heart of our design, taking into account the needs and capabilities of different users (patients, operators, specialists). Our combined expertise in industrial design, interaction design and mechanical engineering guarantees the creation of safe, innovative, high-performance, ergonomic and aesthetically pleasing medical products.
activities
PRODUCT DEVELOPMENT
- Proof of concept
- Industrial design
- Front-end UX/UI design
- Image rendering
- Mechanical engineering
- Mock-ups, functional prototypes, clinical prototypes
- Verification testing (V&V)
- BOM, assembly and manufacturing drawings
In collaboration with the customer’s teams and our ISO 13485 partners:
HUMAN FACTORS (USAGE)
-
- Definition of user requirements
- Risk analysis
- FMEA and FTA studies
- Validation tests (V&V)
- Clinical tests
QUALITY AND REGULATORY MANAGEMENT
- Design inputs and outputs DI/DO
- DHF design history file
- Design reviews
- QMS implementation and hosting via our partners
- Support for regulatory strategy and Health Canada and FDA filings via our partners

Products rooted in reality
Your proof-of-concept partner
The Alto team has the know-how to support the development of your ideas and accelerate the creation of your proof of concept for submission to the FDA and Health Canada. Contextual research, concept development, as well as prototype manufacturing and verification testing, help move even the boldest medical product concepts forward. At the start of the mandate, the designer’s point of view enhances the definition of user needs and design inputs.
Subsequently, several verification and usage tests can be carried out quickly thanks to our in-house prototyping and human factors study capabilities, while our mechanical design team elaborates 3D models and production drawings. During this process, we contribute to the traceability of development activities by complying with your internal Design History File (DHF) documentation processes.Our empathy and field-focused approach enable us to create medical products that don’t just tick off a list of requirements, but actually improve the living conditions of patients and the working conditions of healthcare professionals.
To gain a better understanding of real-life practices, we work closely with hospitals, clinics, universities and incubators. We focus on field observations and interviews with healthcare professionals to define up-to-date use cases. This process enables us to specify the environmental (available space, noise, brightness, etc.) and operational conditions defining the characteristics of your future products.
The various stages of use are specified in the form of storyboards and user journeys, to ensure that all aspects of use are covered, from operation to maintenance. This type of analysis also enables you to anticipate potential usage errors or dangerous situations, thus enriching your risk analysis and ensuring user safety. As we develop the features of your products, we regularly consult subject matter experts to compare our ideas with real-life conditions.


















More projects
Product
Client
Industry
Year

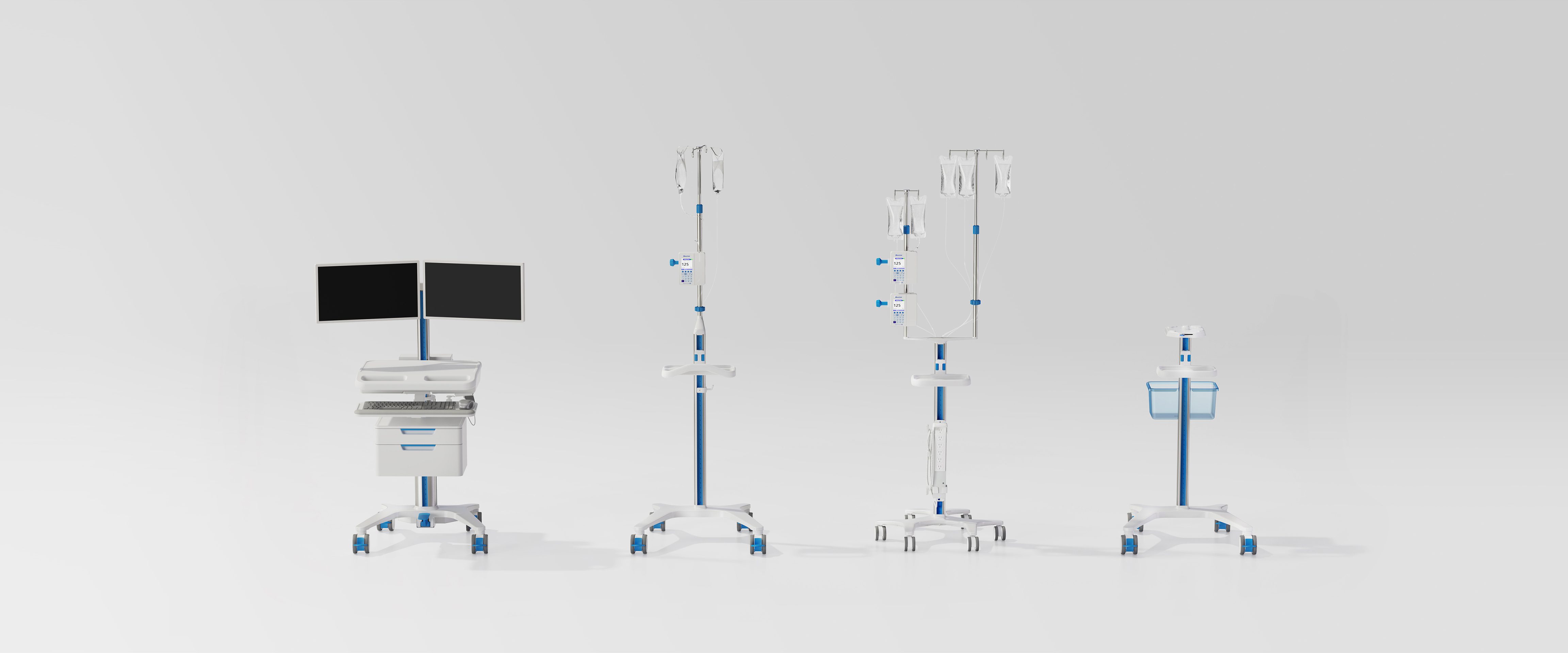
THS product line
TECHNIMOUNT MEDICAL
Medical
2021
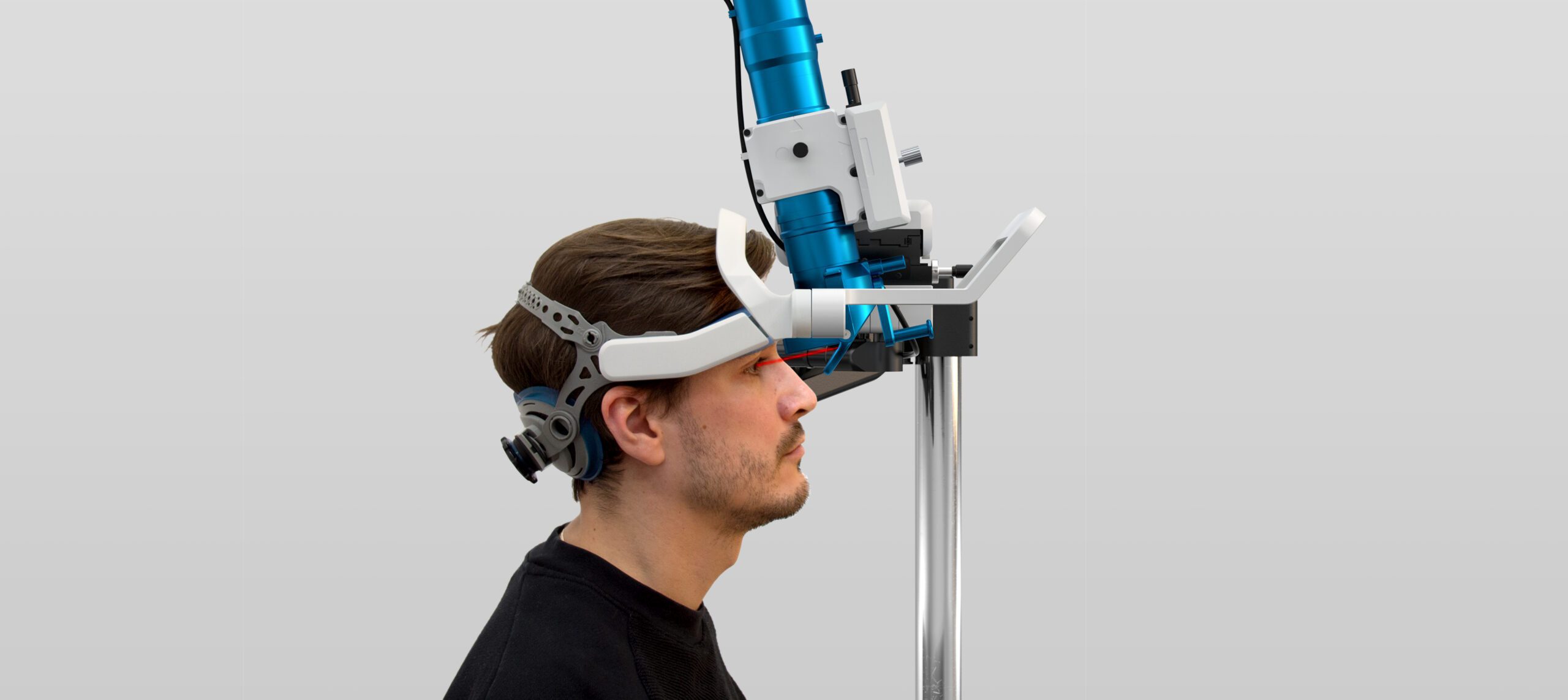
Light distribution system for retinal implants
Nano Retina
Medical
2015

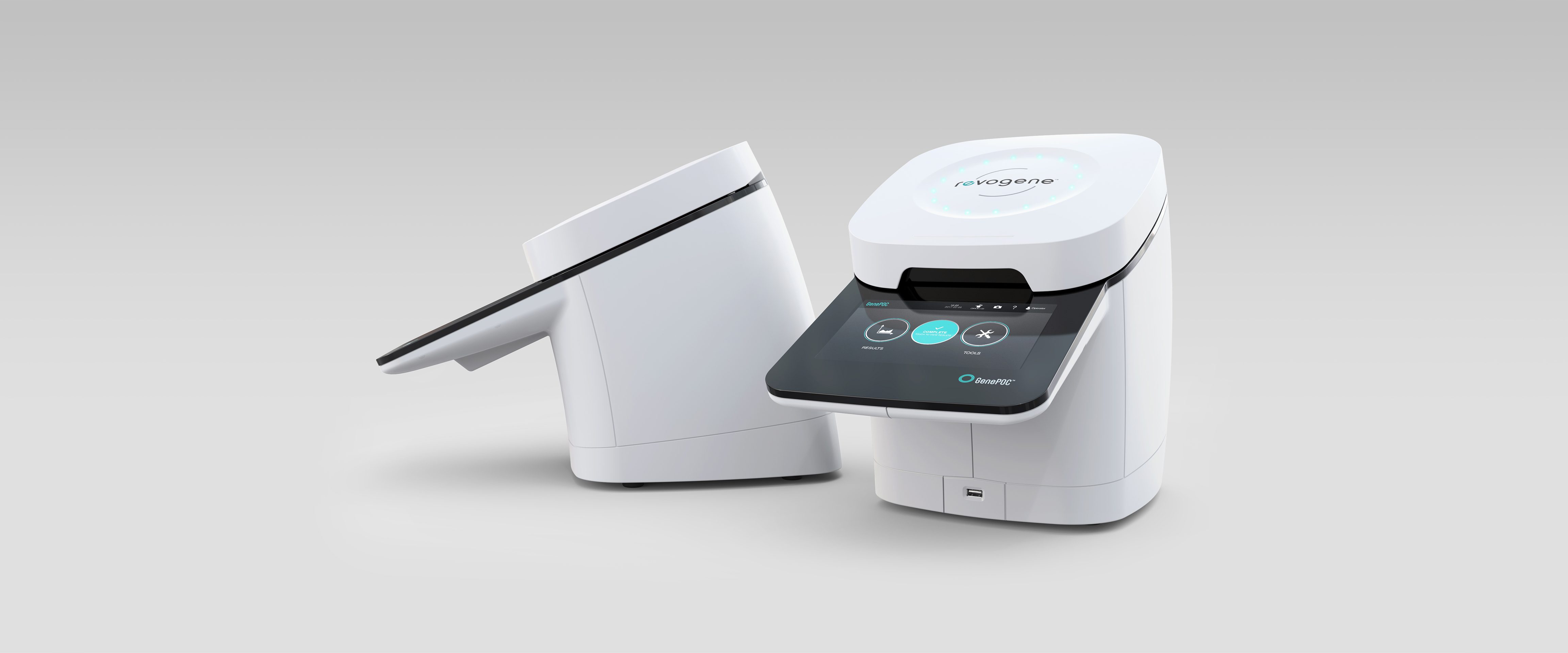
Revogene
Genepoc
Medical
2014
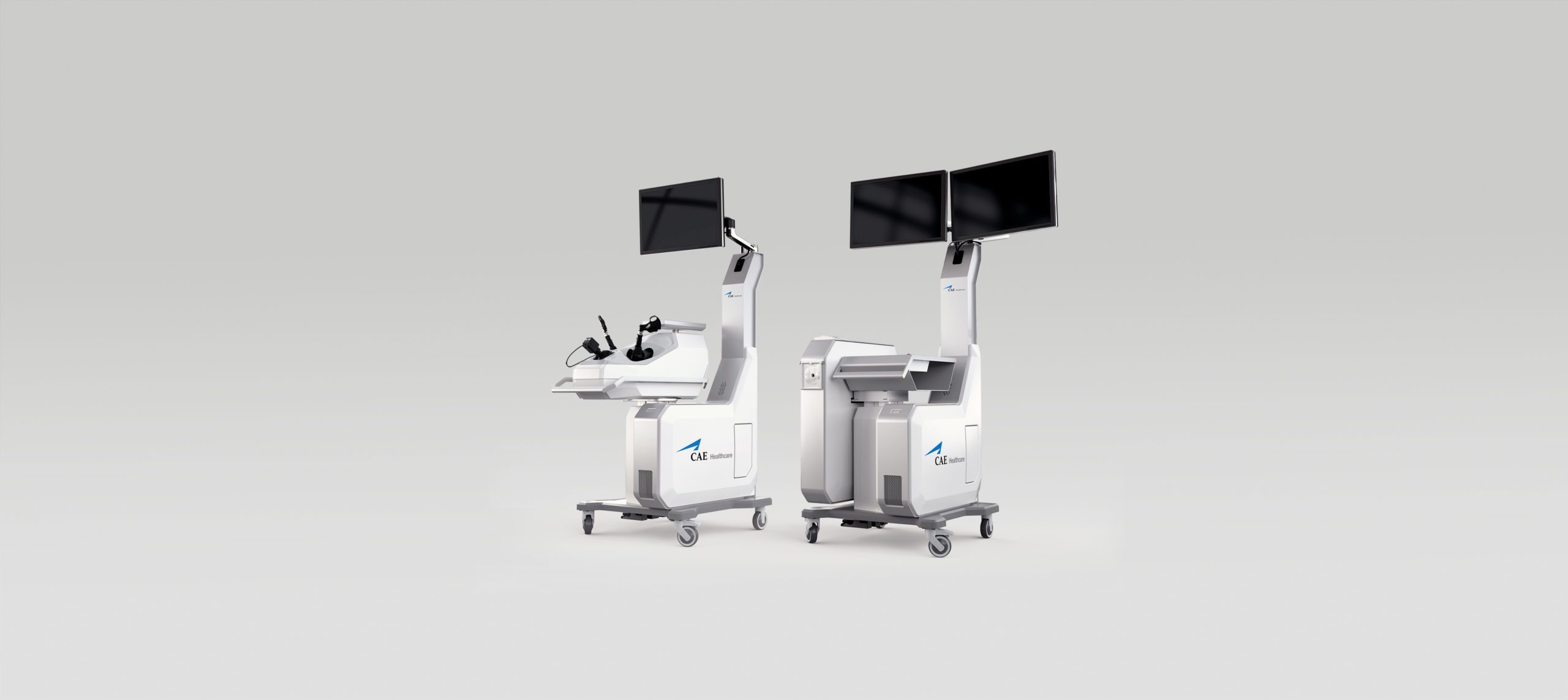
Surgical simulators
CAE Healthcare
Medical
2012

LabPET - Positron emission tomograph
AMI – ADVANCED MOLECULAR IMAGING
Medical
2004